|
The CURDLING of MILK |
| chemistry secrets to obtain the curd |
| |
|
The curdling of milk occurs in different ways with
the help of chemistry let's try to understand. The methods of curdling: ACIDIFICATION: It may occur thanks to milk ferments that transform lactose
in to milk acid, taking the milk to 4.6 ph, or for the addition of acid
substances; RENNET: it is, generally, the sweet curdling that
occurs thanks to enzymes. The substance that in the milk gets the
transformation is casein, a fundamental protein that in the milk is found in
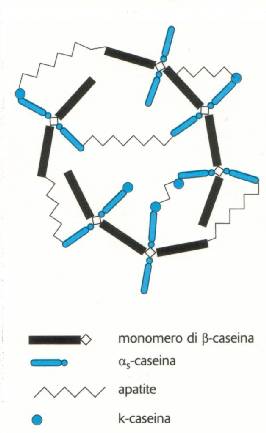
a colloidal solution. The casein is a conjugate protein formed (by beside C,
H, O, N, S) also phosphorus (P) in the form of ester phosphoric acid of which
a part is found inside every unit of a,b,k- "salificato" casein with calcium
and magnesium ions, a part is in the form of tricalcium phospote (apatite)
that joins together the units of a, b, k- casein as in the picture. The whole
casein "micella" is called "phospho-caseinatum of calcium". The
quantity of calcium ions regulates the aggregation's state of "micelle" and the sped of their
flocculation: without calcium casein can't coagulate. ACID CURDLING In normal conditions milk has a ph of about 6.5-6.7
and at this ph value the casein is without protons, it has a negative charge
and therefore the casein micelles are relatively soluble, because they repel
each others. In an acid ambient milk coagulates, because casein has its
isoelectric point at 4.6 ph, that is at this ph value it has a quantity of
positive charges equal to the quantity of negative charges and the positive
part of each "micelle" is attracted by the negative part of the others, causing the
formation of ionic bonds among the "micelle" working against the
dipole-dipole bonds with water, so that the protein precipitates in the form
of demineralized casein and in the solution remain soluble calcium salts. TRADITIONAL CURDLING OF RENNET The rennet (see above) is a substance extracted by
the abomasus of the suckling calf and contains natural "proteolitici" enzymes that
change the structure of casein, that "qelifies" with calcium ions
whose presence is guaranteed the acidification of milk to use. The
fundamental enzyme is the "chimasi" that splits a particolar peptiolic bond, between methionine and
phenylanine that is found in the k-casein and that keeps a hydrophyle
carbohydrate's fragment. After the removal of this fragmet, the regulation
factor in the watery solution is missing and the "micelle", made unstable, in the
presence calcium ions, tend to join, forming the curd called "calcium
para- caseinatum". Unlike the curd obtained by acidification, this keeps
the most part of tricalcium phospate. |
 above:the casein molecule. below:the preparation phases of "presamica" curd.
phases of "presamica"
curdling (according to Mrs Emilia from Abasse)- calf
abomasus containing the curd natural enzymes. |

